Hangzhou and Shaoxing, China, 17 June 2020 -- Ascletis Pharma Inc. (HKEX code:1672) announces today that its partner Sagimet Biosciences Inc (Sagimet, formerly 3-V Biosciences Inc), announced today positive results on oral, once-daily non-alcoholic steatohepatitis (NASH) drug candidate TVB-2640 (Ascletis code: ASC40) from its Phase 2 (FASCINATE-1) clinical trial. The preliminary data showed that ASC40 (TVB-2640) significantly reduced liver fat, the primary efficacy endpoint of this trial, with a 61% responder rate in the 50 mg group. Participants also showed improvement in markers of liver function and fibrosis. Ascletis, through its subsidiary, has an exclusive license to develop, manufacture and commercialize ASC40 (TVB-2640) and related compounds in Greater China. In conjunction with the exclusive license agreement, Sagimet raised US$25 million in a Series E financing led by Ascletis, through its subsidiary, together with new and existing investors.
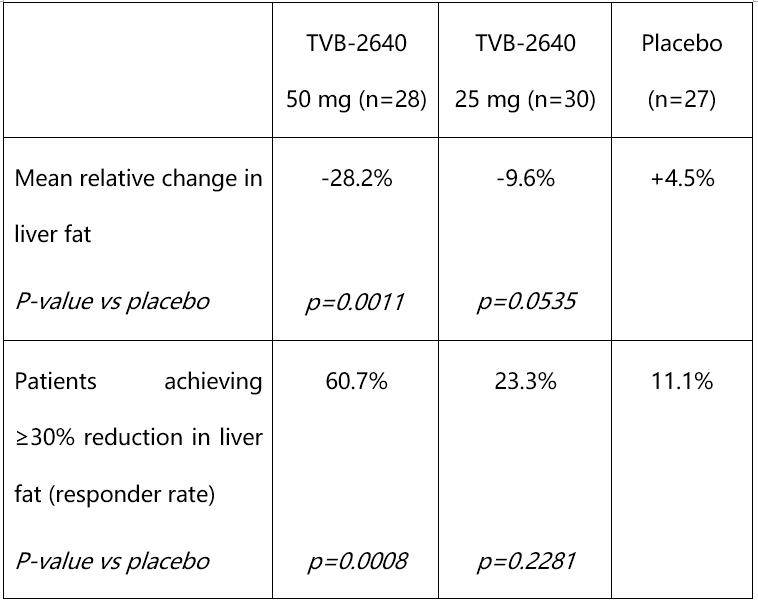
In the Phase 2 (FASCINATE-1) randomized, placebo-controlled trial of 99 patients in the United States, clinicians evaluated the safety and efficacy of oral, once-daily dosing of TVB-2640 for 12 weeks. Study participants were required to have at least 8% liver fat at baseline, as measured by magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), and evidence of stage F1 to F3 liver fibrosis. The study demonstrated a statistically significant dose-dependent, relative reduction in liver fat of 28.2% in the 50 mg group versus an increase of 4.5% in the placebo group. TVB-2640 also significantly decreased ALT by up to 20.4% and LDL-cholesterol by up to 7.6% at week 12. These decreases indicate improved liver function and metabolic health.
TVB-2640 was well-tolerated with a benign adverse event profile, predominantly grade 1 events and no on-treatment serious adverse events.
An additional 50 mg cohort of 25-30 NASH patients in China has started screening.
Ascletis has two additional drug candidates, developed in house, in its NASH pipeline. ASC41 is a highly potent and selective agonist for thyroid hormone receptor beta (THR-beta) and received IND approval from China’s NMPA. Topline results from ASC41 Phase 1 safety, pharmacokinetics and preliminary efficacy (LDL-C) studies are expected by the end of 2020, with Ascletis’ proprietary oral tablet formulation. ASC42 is a pre-IND NASH candidate against a different target. With 3 candidates at various development stages, Ascletis’ NASH pipeline is competitive on the global scale and these three NASH candidates can be used alone or in combination.