Hangzhou and Shaoxing, China, November 7, 2022 -- Ascletis Pharma Inc. (HKEX:1672, “Ascletis”) announces today that the abstract of a Phase I, Single-Dose Study of ASC43F for non-alcoholic steatohepatitis (NASH) has been reported at The Liver Meeting® 2022 of the American Association for the Study of Liver Diseases (AASLD) as poster presentation. The summary of the abstract is shown as below:
Title: A Phase I, Single-Dose Study to Evaluate The Safety, Tolerability, and Pharmacokinetics of ASC43F, A Fixed-Dose Combination Oral Tablet of ASC41, A Thyroid Hormone Receptor Beta Agonist, and ASC42, A Farnesoid X Receptor Agonist in Healthy Subjects
Abstract/Poster number: 2314
Category: NAFLD therapy
Study Design:
ASC43F-101 (NCT05118516) is an open-label, single-dose, Phase I study in healthy subjects. Eight subjects aged 18 to 65 years who weighed at least 50 kg for men, and at least 45 kg for women and had body mass index (BMI) within the range of 18.5-32 kilogram per meter square (kg/m2) were planned to be enrolled in this study. Two eligible subjects would be enrolled first. After the 7-day safety assessment of the first two sentinel subjects and no stopping rule was met, the remaining 6 subjects would be enrolled.
Results:
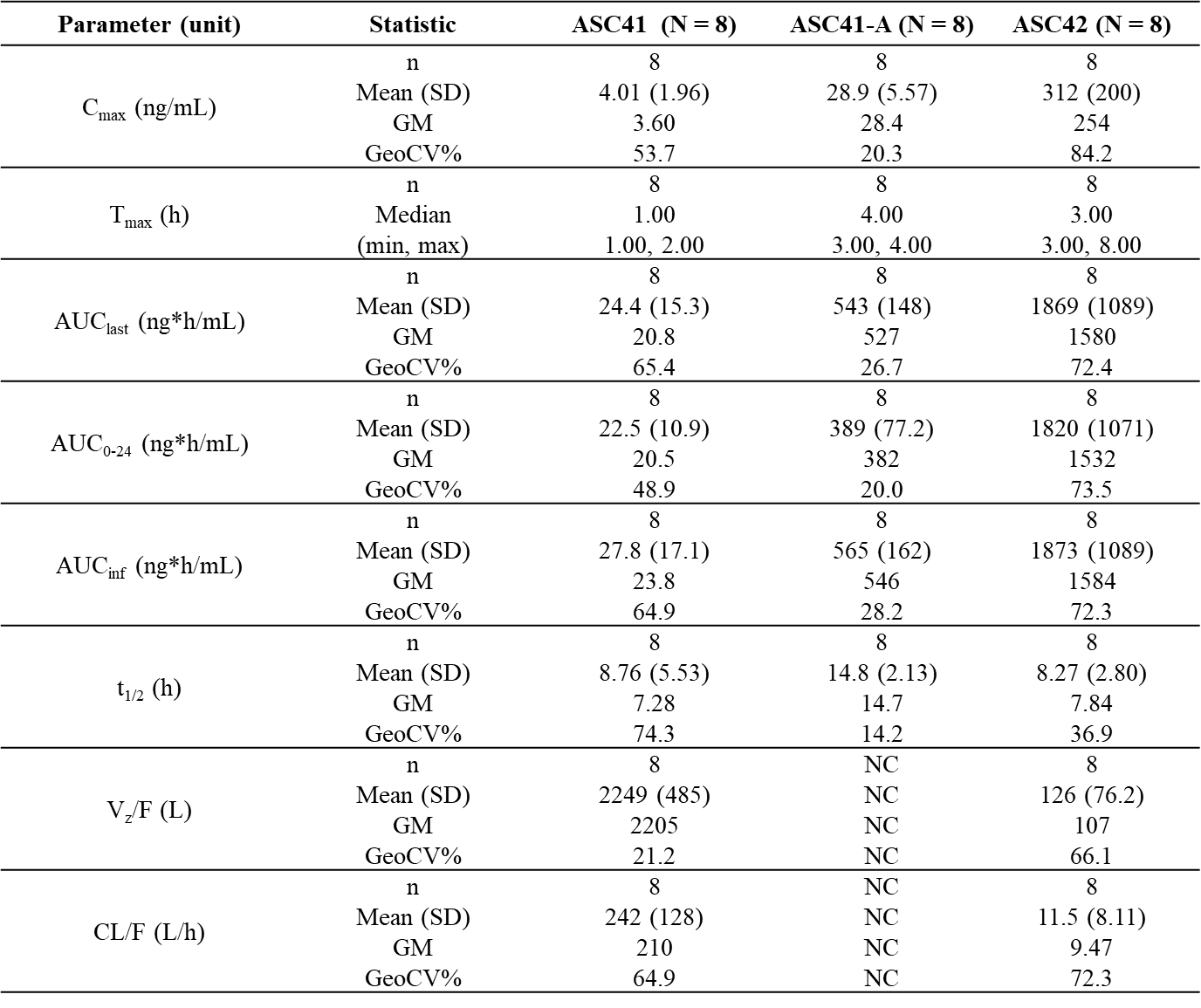
Table 1: Summary of PK parameters of ASC41, ASC41-A, and ASC42 from ASC43F versus monotherapy in healthy subjects
Conclusion
This Phase I study demonstrated that ASC43F showed good tolerability and safety profiles, and pharmacokinetics (PK) parameters of ASC41/ASC41A and ASC42 from ASC43F were similar to those of ASC41 and ASC42 as monotherapy. ASC43F is a one-pill, once-a-day fixed-dose combination (FDC) for NASH treatment, thus will improve patient compliance.